History & Timeline
History and Timeline
1998

Data Management Unit (DMU) was established.
• Professor Emeritus Dr.Dwip Kitayaporn, Head of the Unit
• Knowledge and technical transfer, sponsored by VaxGen Inc, USA
• DataFax system implementation for the phase I/II AIDSVAX® trial
2003
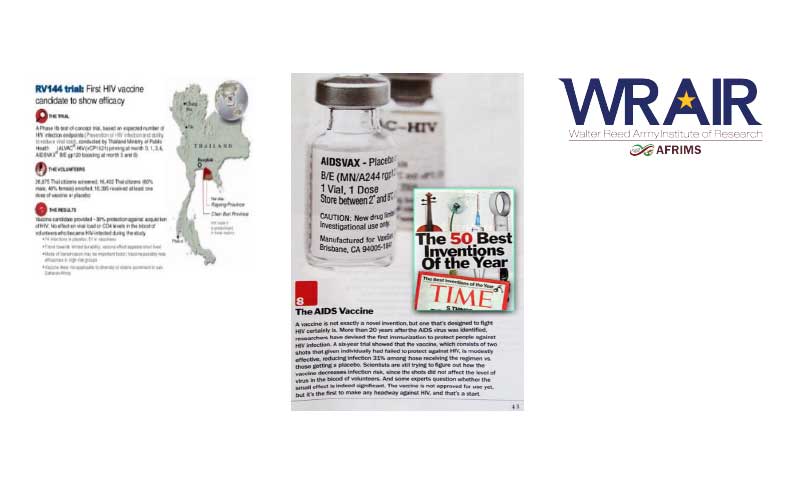
Data management for the RV144 project, the largest Phase III HIV vaccine trial involving 16,000 participants.
• Sponsored by the Surgeon General of the United States Army and conducted by the Ministry of Public Health (Thailand) with support from the United States Army Medical Research and Materiel Command (USAMRDC) and the National Institute of Allergy and Infectious Diseases (NIAID),the National Institutes of Health (NIH)
2008

Center of Excellence for Biomedical and Public Health Informatics (BIOPHICS) was established with the initial support from Rockefeller Foundation.
• Professor Dr. Jaranit Kaewkungwal, Director
• Expand to Public Health Informatics
• Received funding support from Microsoft Research to leverage the smart
phone technology as a health communication tool in the under-served
communities along the Thailand border
• Regional Clinical Data Management of Southeast Asia Influenza Clinical
Research Network (SEA-ICRN)
2009
• Received funding support from the Bill & Melinda Gates Foundation through a collaborative effort between the Bureau of Vector Borne Disease (BVBD), the Ministry of Public Health (Thailand), and WHO to expand the innovative mHealth project using of cell-phone technology to enhance the early disease detection, case investigation, patient compliance and follow-up visits of malarial patients in 7 provinces along Thailand-Cambodia borders

• Data Management and statistical analysis of the series for the GPO Flu Vaccine Trial in collaboration with Vaccine Trial Center (VTC)

2010
A national training center for a 5-month course on Good Clinical Data Management Practice (GCDMP) for data managers, supported by the Thailand Center of Excellence for Life Sciences (TCELS)

2011
• Awarded a Bill & Melinda Gates Foundation “Grand Challenges Explorations” Grant to pursue a prototype for mobile technology applications to improve vaccination strategies for stateless children in hard-to-reach areas to achieve the Expanded Program of Immunization (EPI) coverage targets among the under-served and extremely vulnerable group.

• Received funding support from the Global Fund Round 10 through a collaborative effort with Bureau of Vector Borne Disease (BVBD), the Ministry of Public Health (Thailand) to expand the initiative mHealth project and develop a National Electronic Malaria Information System (eMIS).

2012
• Organized an international training on the Clinical Data Interchange Standards Consortium (CDISC), supported by the Thailand Center of Excellence for Life Sciences (TCELS)
• Started organizing Good Clinical Practice (GCP) and statistical analysis for clinical research training workshops on a biennial basis

2014

Data management and statistical analysis support for the series for the Tetanus-Diphtheria-Acellular Pertussis Vaccine Trial, sponsored by BioNet-Asia. The vaccine is now licensed in Thailand.
2015

Data management and statistical analysis support for Dreamlopments
• M-FUND project, a low-cost not-for-profit health protection scheme for migrants in Thailand
• C-FREE project, the first community-based care model in Thailand for the diagnosis and treatment of hepatitis C in people who inject drugs (PWID)
2017

Amnat Khamsiriwatchara, Head of BIOPHICS
• Received funding support from the Global Fund Round 10 through a collaborative effort with Bureau of Tuberculosis Disease, the Ministry of Public Health (Thailand) to develop the National Tuberculosis Information Program (NTIP) as the central network for the management of TB over 1,200 TB hospitals and clinics nationwide
• Served as Data management core in collaboration with Mahidol-Vivax Research Unit (MVRU) for the Southeast Asia International Centers of Excellence for Malaria Research (ICEMR), funded by the NIH
2021

Data management and statistical analysis support for Covid-19 vaccine trials
• Phase I/II SARS-CoV-2 NDV-HXP-S vaccine trials in collaboration with the Instituto Butantan (Brazil), the Government Pharmaceutical Organization (Thailand) and Institute of Vaccines and Medical Biologicals (Vietnam), supported by PATH
• Phase I/II/III SARS-CoV-2 mRNA vaccine involving 20,000 participants in Vietnam, sponsored by Biocare Vietnam Biotehnology JSC
• Phase I/II SARS-CoV-2 Ad26.COV2.S vaccine in Thailand, sponsored by Janssen
• Phase I/II SARS-CoV-2 mRNA ChulaCOV19 Vaccine Trials in Australia and Thailand, sponsored by Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand



